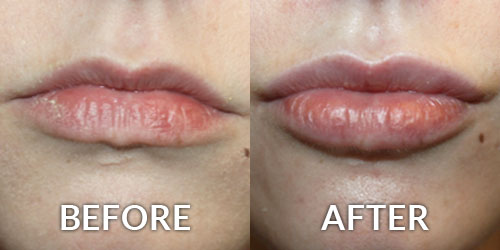
On June 1, 2016 Allergan announced it received approval from the U.S. Food and Drug Administration (FDA) to market Juvederm Volbella XC, a filler developed specifically to increase fullness of the lips and soften the appearance of the lines around the mouth, known as perioral rhytids.
Similar to Juvederm Voluma XC, Juvederm Volbella XC is formulated with VYCROSS, a proprietary filler technology from Allergan that is comprised of a combination of low and high molecular weight hyaluronic acid (HA), a naturally occurring substance found in the body. Although HA is contained in almost every cell, fifty percent of the body’s hyaluronic acid is concentrated in the skin, resulting in elasticity and smoothness. The skin’s production of HA decreases with age, contributing to wrinkling and loss of facial volume. VYCROSS technology was first introduced in this country with the 2013 FDA approval of Allergan’s Juvederm Voluma XC, a filler for age-related mid-face volume loss
A product unlike anything currently available in the United States, Juvederm Volbella XC is a smooth gel suitable for adding subtle volume to the lips and reducing the appearance of perioral lines. Unlike Juvederm Voluma XC, which is denser and used to produce substantial volume, Volbella XC will be used for more subtle corrections as well as more superficial corrections. The patented technology blends different molecular weights of hyaluronic acid which contributes to the gel’s duration, with results lasting up to one year or more.
Board Certified Plastic Surgeon Brian S. Glatt, MD, FACS looks forward to providing this innovative treatment to his patients. Juvederm Volbella XC is expected to be available in October 2016. To schedule an appointment with Dr.Glatt please call the Morristown office of Premier Plastic Surgery Center of New Jersey at 973-889-9300.